VE202

VE202 for Inflammatory Bowel Disease (IBD)
VE202 is a wholly-owned, orally-administered defined bacterial consortium candidate consisting of 16 strains of live commensal bacteria for the treatment of IBD.

Role of gut dysbiosis in IBD
Despite progress in understanding IBD pathways, what causes the disease has not been clearly established. The most widely accepted view is that IBD results from altered interactions between the gut microbiota and the immune system in genetically susceptible hosts.
Yet, all approved IBD therapies aim at suppressing the immune system and ignore the microbiome as a disease driver. Correcting gut dysbiosis is a missing piece in the current treatment of IBD.
Our therapeutic approach with VE202
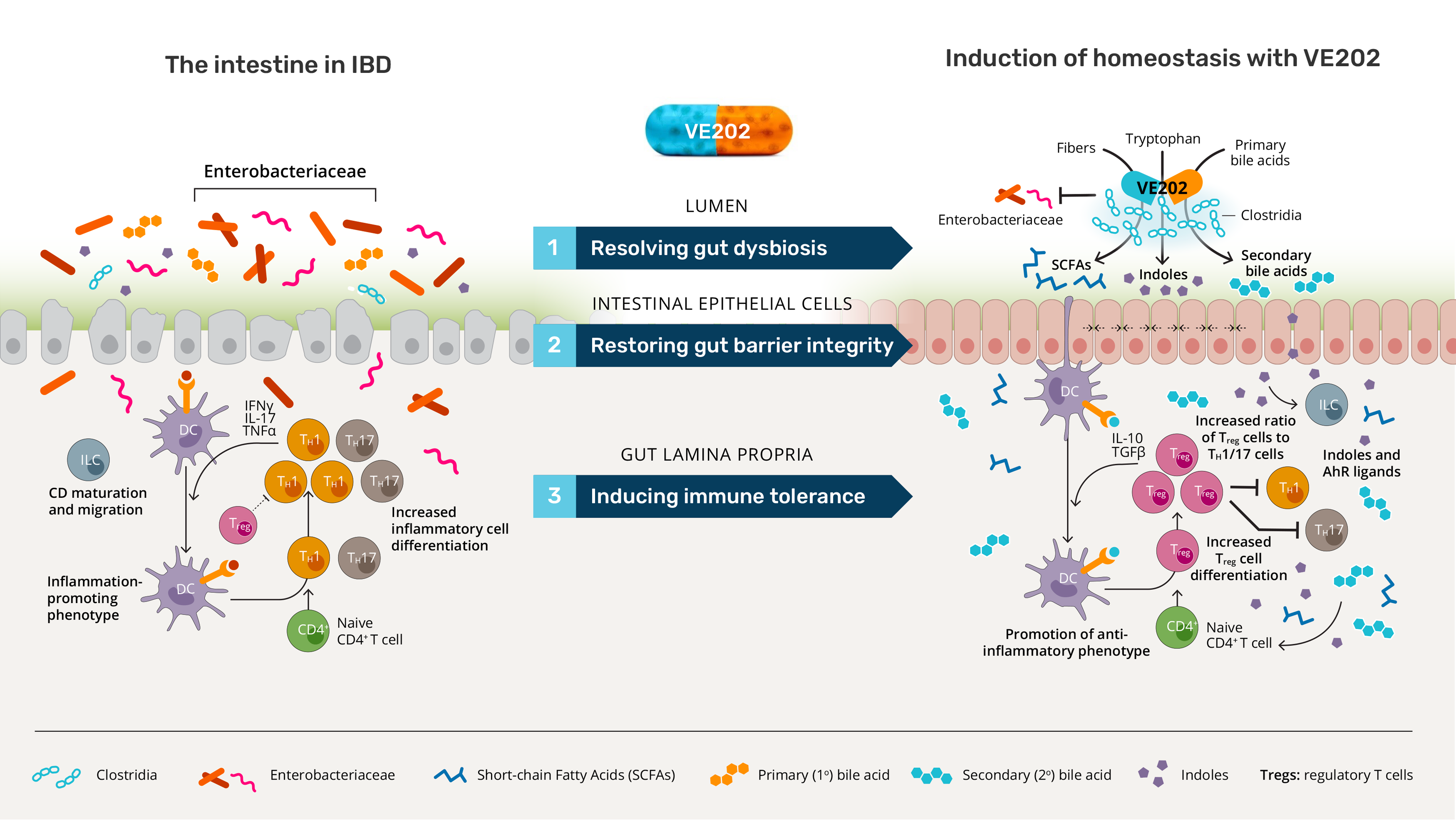
In contrast to existing therapies, which are directed at inhibiting an overactive immune response, the pleiotropic mechanisms of action of VE202 have the potential to address multiple contributors to IBD: gut dysbiosis, gut barrier damage, and immune dysregulation.
The strains in VE202 have been selected for their ability to restore intestinal homeostasis in IBD, by:
- Resolving gut dysbiosis: VE202 strains reduce colonization by pro-inflammatory Enterobacteriaceae that are implicated in IBD relapses
- Restoring gut barrier integrity: VE202 strains enhance markers of gut barrier integrity
- Inducing immune tolerance: VE202 strains modulate the balance of Treg cells and Th17 cells in the intestine, inducing the restoration of a tolerogenic environment.
Phase 1 clinical trial highlights
VE202 was well tolerated in a completed Phase 1 study and observed to be non-immunosuppressive. Adverse events were generally mild, gastrointestinal in nature, and transient; a very limited number of adverse events, none of which were serious, were related to VE202.
Phase 2 clinical trial highlights
The Phase 2 study of VE202 did not meet its primary efficacy endpoint. Observed response rates in the VE202 group were not statistically different from those in the placebo group. VE202 was generally safe and well tolerated, with no reports of treatment-related serious adverse events.
Clinical Trial
Clinical Research Study for Ulcerative Colitis
We are currently conducting COLLECTiVE202, a randomized, double-blind, placebo-controlled, multinational Phase 2 study of VE202 in individuals with mild-to-moderate UC. The FDA has granted VE202 Fast Track designation for the treatment of adults with UC.